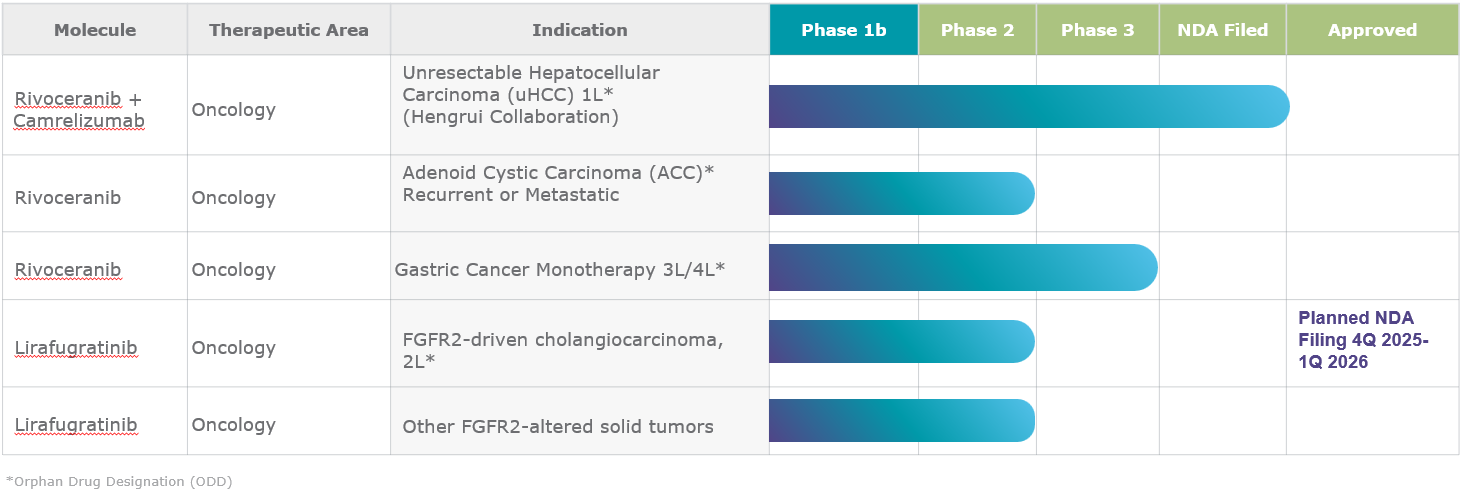
CLINICAL STUDIES
Currently, rivoceranib is being studied both as a monotherapy and in combination with chemotherapy and immunotherapy.1
Clinical studies are planned or underway in multiple tumor types including:
- Hepatocellular carcinoma (in combination with camrelizumab)
- Adenoid cystic carcinoma
- Colorectal cancer (in combination with LONSURF®)
- Gastric cancer
Orphan drug designations for rivoceranib have been granted in gastric cancer (in the US, EU, and South Korea), in adenoid cystic carcinoma (in the US), and hepatocellular carcinoma (in the US).1
All product and company names are trademarks™ or registered® trademarks of their respective holders. Use of them does not imply any affiliation with or endorsement by them.
*Breakthrough therapy designation granted for cholangiocarcinoma.
Reference: 1 Markets Insider. Elevar Therapeutics Receives Orphan Drug Designation from FDA for Rivoceranib for the Treatment of Hepatocellular Carcinoma (HCC). Published November 11, 2021. Accessed June 1, 2023. https://markets.businessinsider.com/news/stocks/elevar-therapeutics-receives-orphan-drug-designation-from-fda-for-rivoceranib-for-the-treatment-of-hepatocellular-carcinoma-hcc-1030965744


